


LACTOSE INTOLERANCE: THE MOST COMMON ENZYME INTOLERANCE
Lactose is a disaccharide, present in solution in the milk of mammals and its derivatives, where it represents about 98% of sugars. After its intake by the diet, lactose is hydrolyzed in the duodenum by the enzyme lactase (a protein present on the brush border of intestinal enterocytes) that cleaves it into two sugars: galactose and glucose. The two sugars are absorbed in the intestine and through passive diffusion are released into the blood stream from the enterocytes. They then reach the liver where galactose is converted to glucose. Lactose intolerance is the most frequent food intolerance in the whole world.
Causes:
Lactose intolerance can be generated by the following conditions:
1. Congenital lactase deficiency: present since birth, it is a very rare event caused by the inability of the newborn to produce lactase (genetic basis).
2. Primary lactase deficiency: caused by a deficient production of lactase by the enterocytes. All children up to approximately two years of age produce lactase to digest breast milk: during weaning, in intolerant children, the enzyme is no longer produced or produced in gradually lower amounts until adulthood is reached.
3. Secondary lactase deficiency: patients, suffering from diseases causing damages to the small intestine and, therefore, to the cells producing lactase (e.g. Crohn’s disease, celiac disease, intestinal inflammation and infections), may present with a secondary (acquired) intolerance. This condition may be seen on a temporary basis (e.g. viral or bacterial infections), and then disappear when the infection is over (e.g. Rotavirus infections).
Primary and secondary deficiencies are characterised by a deficient production of the enzyme by enterocytes. The undigested lactose in the gut is then metabolized by intestinal bacteria with the production of gases (such as C02, H2, CH4) and an osmotic effect with H2O recall, which lead to the typical symptoms of lactose intolerance described above (diarrhea, nausea, bloating, cramps, intestinal disorders). Sometimes, few volatile fatty acids are produced leading to a decrease of the stool pH.
Biomolecular Mechanisms Regulating Lactose Metabolism
Based on the ability to digest lactose or not, two phenotypes can be found in the human population:
• Lactase Non-Persistent LNP (intolerance): The phenotype is characterized by a time-based decrease of lactase production: this is the ancestral condition of mankind. After weaning, the enzyme is no longer produced, or is produced in gradually more and more lower quantities until adulthood.
• Lactase Persistent LP (tolerant): This phenotype is characterized by the ability to produce lactase even in adulthood. It appeared about 10,000 years ago with the development of animal breeding. The appearance of lactase persistence, due to a mutation occurred in the MCM6 gene upstream of the LCT gene encoding lactase, was immediately a positive benefit, since it allowed to use milk as a food source for the human being during his whole life and not in the post-natal period only.
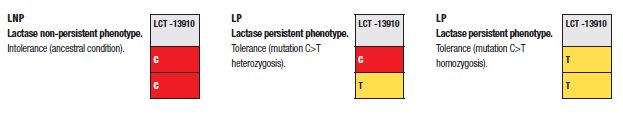
Lactase is a protein of the enterocyte membrane encoded by the LCT gene located on chromosome 2. The low ability to hydrolyze lactose is due to a phenomenon of programmed up-regulation involving the MCM6 gene, upstream of LCT. This gene shows several single base nucleotide polymorphisms with a high association to lactase-persistence. As an example, a genotype presenting with two Thymine (TT) in position -13910 determines Lactase-Persistence (LP), as well as a heterozygous Thymine / Cytosine (T/C) genotype, while a homozygous CC genotype at position -13910 is specific for Lactase not Persistent (LNP) phenotype.1
| LactoGen - code 9241 |

